UG Healthcare is dedicated to consistently delivering excellence through our integrated own brand manufacturing (“OBM”) supply chain. This commitment enables us to serve a diverse customer base across various industries, which has placed their trust and loyalty in our proprietary UNIGLOVES® branded products. We operate a globally owned and managed distribution network, catering to both developed and developing countries.
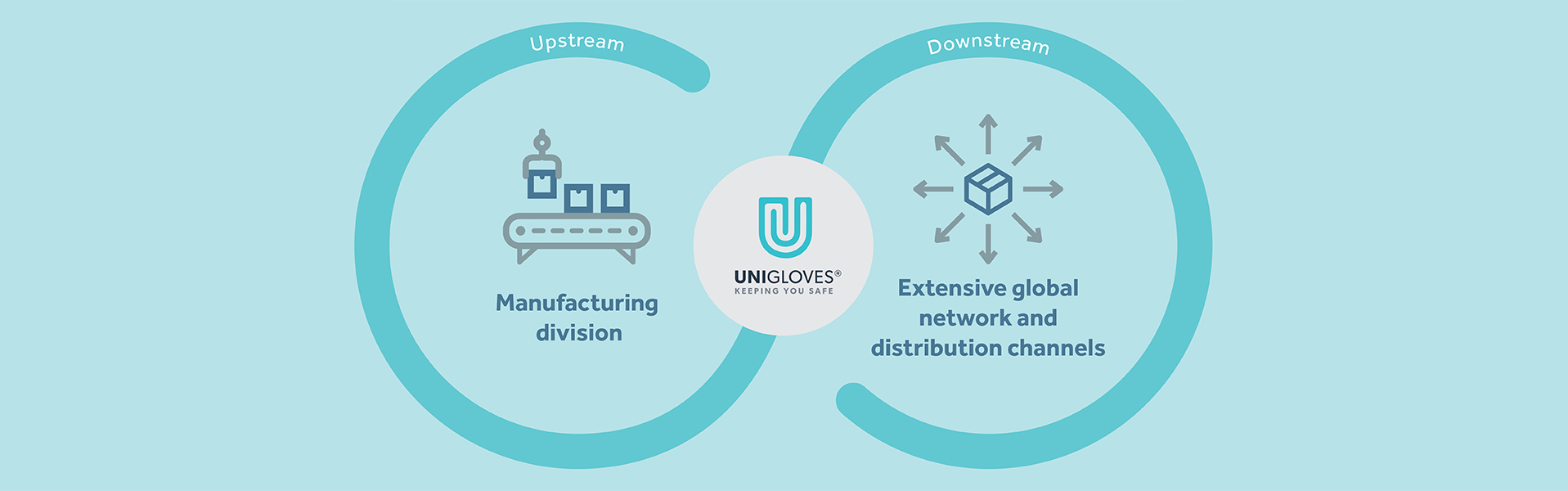
Our integrated OBM supply chain business model, encompassing both upstream manufacturing and downstream distribution for our proprietary UNIGLOVES® branded products, particularly in our premium-quality hand protection solutions, as well as our non-glove hygiene and healthcare ancillary products, enables us to exert greater control over the quality and reliability of our product offerings. These products are essential for end users who require protection against cross-infection and adherence to stringent hygiene standards. We remain committed to addressing the evolving requirements of our end customers across a wide range of industries by providing appropriate hand protection solutions, as well as non-glove hygiene and healthcare ancillary products customised for their applications in their professions, thereby keeping them safe.
The Group believes the success of our integrated OBM supply chain business is inherently intertwined with the success of our customers.






OUR BUSINESSES
Our proprietary UNIGLOVES® branded products continue to conform to various international standards and requirements. These include the ASTM International (formerly known as American Society for Testing and Materials), the European standard for medical gloves (“EN455”), ISO 11193 standards (International Organization for Standardization for Single-use medical examination gloves), ISO 10993 standards (International Organization for Standardization for biological evaluation of medical devices), CE and UKCA Type Examination Certificates of Latex and Nitrile Examination Gloves PPE 2016(425) Cat III, the Acceptance Quality Level requirements under the Food and Drug Administration (“USFDA”), the National Medical Products Administration (“NMPA”), the Brazilian Health Regulatory Agency (“ANVISA”), Certificates of Approval (“CA”) under the Department of Occupational Safety and Health (“DSST”) of the Brazil Ministry of Labor and Employment (“MTE”), the National Institute of Metrology, Standardization and Industrial Quality (“INMETRO”), the National Agency for Food and Drug Administration and Control (“NAFDAC”), and the Medical Device Registration Certificate under the Malaysia Medical Device Authority (“MDA”).